GW100
This is a benchmark of G0W0 on 100 molecules, introduced in [1]. For each molecule, the vertical ionization potential (VIP) and the vertical electron affinity (VEA) are computed with the WEST code. Results are compared with corresponding values obtained using other implementations of the G0W0 method and other codes (see list below).
List of codes included in this benchmark:
| code | description | license |
|---|---|---|
| FHI-aims | LO, AE | AC |
| TURBOMOLE | LO, PSP, AE | C |
| BerkeleyGW | AE, PSP, PW, LO, RS | BO |
| VASP | PSP, PW | C |
| WEST | PSP, PW | GO |
Description legend
- AE: All electron
- LO: Localized orbitals
- PSP: Pseudopotentials
- PW: Plane waves
- RS: Real space
License legend
- A: Academic
- B: BSD
- C: Commercial
- G: GPL
- O: Open source
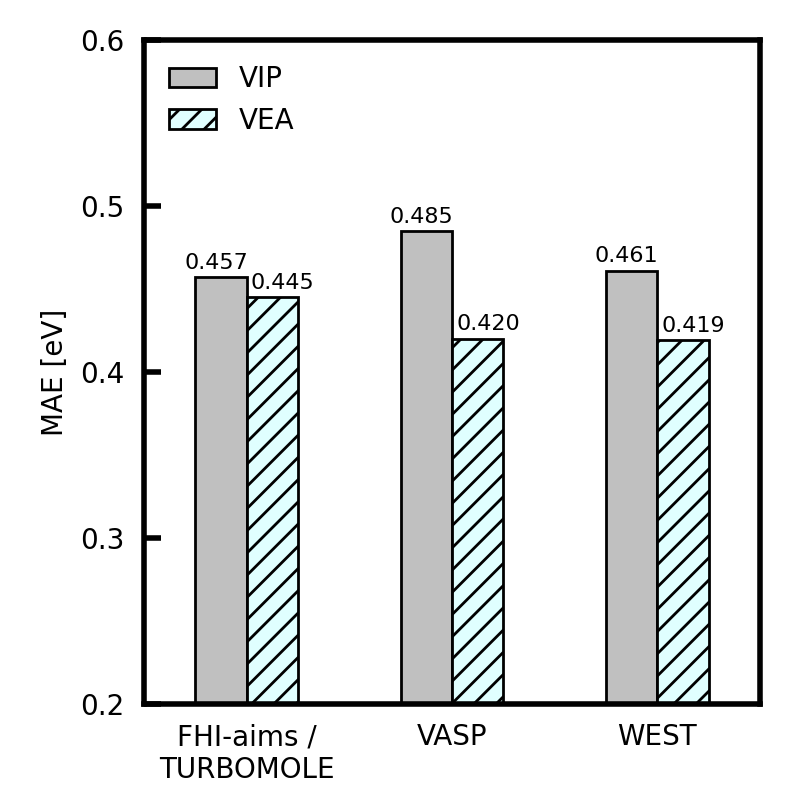
Benchmark summary
The figure below reports the mean absolute error (MAE) in the VIPs or VEAs with respect to experiment. Values of WEST are taken from [1]. Values of FHI-aims and TURBOMOLE are taken from [2]. Values of VASP are taken from [3]. The VIP is computed from the quasiparticle energy of the highest occupied molecular orbital (HOMO). The VEA is computed from the quasiparticle energy of the lowest unoccupied molecular orbital (LUMO). Experimental results are taken from [4].
List of all molecules
Click the molecule name to see the dielectric screening, spectral function, VIP, and VEA computed with the WEST code.
| name | formula | CAS number | picture | |
|---|---|---|---|---|
| 1 | ethylbenzene | C8H10 | 100-41-4 |  |
| 2 | ozone | O3 | 10028-15-6 |  |
| 3 | boron nitride | BN | 10043-11-5 |  |
| 4 | butane | C4H10 | 106-97-8 |  |
| 5 | toluene | C7H8 | 108-88-3 |  |
| 6 | phenol | C6H6O | 108-95-2 |  |
| 7 | pyridine | C5H5N | 110-86-1 |  |
| 8 | tetracarbon | C4 | 12184-80-4 |  |
| 9 | diphosphorous | P2 | 12185-09-0 |  |
| 10 | silver dimer | Ag2 | 12187-06-3 | 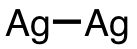 |
| 11 | copper dimer | Cu2 | 12190-70-4 | 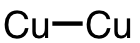 |
| 12 | carbon dioxide | CO2 | 124-38-9 | 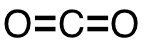 |
| 13 | beryllium monoxide | BeO | 1304-56-9 | 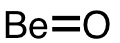 |
| 14 | magnesium monoxide | MgO | 1309-48-4 |  |
| 15 | borane | BH3 | 13283-31-3 |  |
| 16 | dihydrogen | H2 | 1333-74-0 |  |
| 17 | boron monofluoride | BF | 13768-60-0 |  |
| 18 | lithium dimer | Li2 | 14452-59-6 |  |
| 19 | pentasilane | Si5H12 | 14868-53-2 |  |
| 20 | disilane | Si2H6 | 1590-87-0 |  |
| 21 | carbon monoxide selenide | COSe | 1603-84-5 |  |
| 22 | gallium monochloride | GaCl | 17108-85-9 |  |
| 23 | phosphorus nitride | PN | 17739-47-8 |  |
| 24 | diborane | B2H6 | 19287-45-7 |  |
| 25 | diarsenic | As2 | 23878-46-8 |  |
| 26 | sodium dimer | Na2 | 25681-79-2 |  |
| 27 | potassium dimer | K2 | 25681-80-5 |  |
| 28 | rubidium dimer | Rb2 | 25681-81-6 |  |
| 29 | hydrazine | N2H4 | 302-01-2 |  |
| 30 | hexafluorobenzene | C6F6 | 392-56-3 |  |
| 31 | sodium tetramer | Na4 | 39297-86-4 |  |
| 32 | sodium hexamer | Na6 | 39297-88-6 |  |
| 33 | carbon monoxide sulfide | COS | 463-58-1 |  |
| 34 | formaldehyde | H2CO | 50-00-0 |  |
| 35 | carbon tetraiodide | CI4 | 507-25-5 |  |
| 36 | cyclopentadiene | C5H6 | 542-92-7 |  |
| 37 | copper monocyanide | CuCN | 544-92-3 |  |
| 38 | carbon tetrabromide | CBr4 | 558-13-4 |  |
| 39 | carbon tetrachloride | CCl4 | 56-23-5 |  |
| 40 | urea | CH4N2O | 57-13-6 |  |
| 41 | bromoethylene | C2H3Br | 593-60-2 |  |
| 42 | iodoethylene | C2H3I | 593-66-8 |  |
| 43 | diethylether | (C2H5)2O | 60-29-7 |  |
| 44 | aniline | C6H5NH2 | 62-53-3 |  |
| 45 | cyclooctadiene | C8H8 | 629-20-9 |  |
| 46 | carbon monoxide | CO | 630-08-0 |  |
| 47 | ethanol | CH3CH2OH | 64-17-5 |  |
| 48 | formic acid | HCOOH | 64-18-6 |  |
| 49 | thymine | C5H6N2O2 | 65-71-4 |  |
| 50 | uracil | C4H4N2O2 | 66-22-8 |  |
| 51 | methanol | CH3OH | 67-56-1 |  |
| 52 | cytosine | C4H5N3O | 71-30-7 |  |
| 53 | benzene | C6H6 | 71-43-2 |  |
| 54 | adenine | C5H5N5 | 73-24-5 |  |
| 55 | guanine | C5H5N5O | 73-40-5 |  |
| 56 | methane | CH4 | 74-82-8 |  |
| 57 | ethane | C2H6 | 74-84-0 |  |
| 58 | ethylene | C2H4 | 74-85-1 |  |
| 59 | acetylene | C2H2 | 74-86-2 |  |
| 60 | hydrogen cyanide | HCN | 74-90-8 |  |
| 61 | propane | C3H8 | 74-98-6 |  |
| 62 | krypton | Kr | 7439-90-9 |  |
| 63 | neon | Ne | 7440-01-9 |  |
| 64 | argon | Ar | 7440-37-1 |  |
| 65 | helium | He | 7440-59-7 |  |
| 66 | xenon | Xe | 7440-63-3 |  |
| 67 | sulfur dioxide | SO2 | 7446-09-5 |  |
| 68 | chloroethylene | C2H3Cl | 75-01-4 |  |
| 69 | fluoroethylene | C2H3F | 75-02-5 |  |
| 70 | acetaldehyde | CH3CHO | 75-07-0 |  |
| 71 | carbon disulfide | CS2 | 75-15-0 | 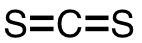 |
| 72 | cyclopropane | C3H6 | 75-19-4 |  |
| 73 | carbon tetrafluoride | CF4 | 75-73-0 | 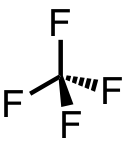 |
| 74 | diiodide | I2 | 7553-56-2 | 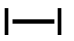 |
| 75 | lithium monohydride | LiH | 7580-67-8 |  |
| 76 | hydrogen chloride | HCl | 7647-01-0 | 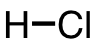 |
| 77 | sodium monochloride | NaCl | 7647-14-5 |  |
| 78 | hydrogen fluoride | HF | 7664-39-3 |  |
| 79 | ammonia | NH3 | 7664-41-7 |  |
| 80 | potassium monohydride | KH | 7693-26-7 |  |
| 81 | hydrogen peroxide | H2O2 | 7722-84-1 |  |
| 82 | dibromide | Br2 | 7726-95-6 |  |
| 83 | dinitrogen | N2 | 7727-37-9 |  |
| 84 | water | H2O | 7732-18-5 |  |
| 85 | potassium monobromide | BrK | 7758-02-3 |  |
| 86 | difluoride | F2 | 7782-41-4 |  |
| 87 | dichloride | Cl2 | 7782-50-5 |  |
| 88 | germane | GeH4 | 7782-65-2 |  |
| 89 | hydrazoic acid | HN3 | 7782-79-8 |  |
| 90 | hydrogen sulfide | SH2 | 7783-06-4 |  |
| 91 | magnesium difluoride | MgF2 | 7783-40-6 |  |
| 92 | sulfur tetrafluoride | SF4 | 7783-60-0 |  |
| 93 | titanium tetrafluoride | TiF4 | 7783-63-3 |  |
| 94 | aluminum trifluoride | AlF3 | 7784-18-1 |  |
| 95 | aluminum triiodide | AlI3 | 7784-23-8 |  |
| 96 | arsine | AsH3 | 7784-42-1 |  |
| 97 | magnesium dichloride | MgCl2 | 7786-30-3 |  |
| 98 | lithium monofluoride | LiF | 7789-24-4 |  |
| 99 | phosphine | PH3 | 7803-51-2 |  |
| 100 | silane | SiH4 | 7803-62-5 |  |
References
- GW100: Benchmarking G0W0 for Molecular Systems, M.J. van Setten et al., J. Chem. Theory Comput. 11, 5665 (2015).
- GW100: A Plane Wave Perspective for Small Molecules, E. Maggio et al., J. Chem. Theory Comput. 13, 635 (2017).
- GW100: Comparison of Methods and Accuracy of Results Obtained with the WEST Code, M. Govoni, and G. Galli, J. Chem. Theory Comput. 18, 1895 (2018).
- P.J. Linstrom and W.G. Mallard, Eds., NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg MD, 20899.